
I’ve been hearing the acronym TPD bandied about by a lot of vapers recently – particularly on YouTube.
The assumption is, I guess, that every single vaper out there knows all there is to know about the Tobacco Products Directive and what it means to you the vaper – the e-liquid producers – the manufacturers – social media – the reviewers and even to us at EcigClick.co.uk.
We had an old saying in newspapers – ‘to assume makes an ass of u and me’.
Childish maybe – but as a journalist it was drilled into me never to assume anything.
To that end we felt it was about the right time to set out exactly what the TPD is and more importantly how it will restrict the way you as individuals choose to vape.
I’ve also heard folks saying ‘it’s a good thing’ or ‘we need legislation’ and then in the next breath adding the rider ‘but I think it’s ridiculous we can’t buy 30ml bottles of juice anymore.’
Look you can’t cherry pick the best bits that suit you as an individual when it comes to legislation of any kind.
The law is the law is the law and that’s it.
I can remember visiting Vapefest last summer and witnessing first hand apathy from vapers who were uninterested in the fight to block TPD.
And I also heard the very real fear from very many e-liquid producers – particularly the smaller brands – who were worried the TPD would spell disaster and see them losing everything they’d invested into their fledgling businesses.
So as the clock ticks down to May 20th 2017 – the date EU legislation on all things vape kicks in – let’s break the TPD down and find out just why so many of us fought against it and hoped that Brexit would unshackle us from it’s ridiculous constraints.
So What Exactly is the TPD? A Brief History
The Tobacco Product Directive is a piece of legislation that basically looks at tobacco control.
And yes the European Union [EU] in its collective ‘wisdom’ has decided that any vaping product or e-cigarette has to be – by law – lumped in as a ‘tobacco product’.
For instance one of the laws is to ban flavoured tobacco across the EU – this includes menthol cigarettes for instance.
Another is to apply the huge and at times frightening warning labels on all tobacco products on sale in the EU.
Given vaping equipment is classed as tobacco that’s why many recent mods and kit packaging has included that nicotine warning label.
Now I could go into the difference between an empty tank or kit and one filled with nicotine and the absurdity of the whole thing – but I’ll leave you to judge that aspect of the TPD!
The first record I can find of TPD legislation appears in June 2001 and it predominantly looked at the manufacture – sale and presentation of tobacco products within the EU.
Things were pretty quiet for a few years until the EU decided there was a need for stronger laws governing the sale of tobacco and in December 2012 fresh laws were written up.
The EU’s Regulation of E-Cigarettes
The EU was concerned about ‘strongly flavoured tobaccos’ entering the market and also voiced concerns over what they described as ‘novel products’ or as we know them e-cigarettes.
From that moment on the writing was on the wall – e-cigarettes and vaping was under the EU spotlight.
To bolster the need for new regulations the EU went on an anti-tobacco campaign including the release of info-graphics such as the one above showing the harm tobacco was doing to the EU population.
There’s no doubt it makes shocking reading – and at this stage there’s no mention of e-cigarettes and their benefits.
The public first really became aware of new legislation surrounding e-cigarettes and vaping with the release of the DIRECTIVE 2014/40/EU.
This is where we see the TPD including what the EU was now calling ‘related products’- related that is to tobacco.
Yeah I know – vaping isn’t a tobacco product – but try telling the politicians and their handpicked scientists that!
And that was pretty much that.
Sure pressure groups sprang up to tackle the absurdity of lumping vaping in with smoking and despite last ditch attempts to block the inclusion of vaping within the TPD the new rules were passed into European law.
In a nutshell the new additions to the TPD were aimed at:
- Minimum standards for the safety and quality of all e-cigarettes and refill containers (otherwise known as e-liquids)
- Information will be provided to consumers so that they can make informed choices
- An environment that protects children from starting to use these products.
Nothing wrong with the sentiment behind those – however the devil is in the detail and it is those details that have and will impact on you as a vaper – which we shall look at soon.
Here in the UK the Government had no choice but to accept this and released The Tobacco and Related Products Regulations 2016.
You can read this HERE
This new updated directive [TPD] came into force last May – which gave vape manufacturers and e-liquid companies a year to comply.
This is why you’ve been able to buy whatever size e-liquid or tank you like this past 12 months – however on May 20th this year [2017] – all that will end.
Online stores and bricks and mortar shops within the EU will have to remove from sale everything that is not TPD compliant at close of play on May 19th it’s as simple as that.
So let’s look at just how the TPD is going to affect you the vaper.
BTW should you wish to read the full TPD – you can do so HERE
The TPD and the Consumer
This is what the new legislation will mean to you the vaper.
I’ll list the individual ‘rules’ and add an opinion on each one.
Just like a review this is my personal opinion based on well over a year of researching and writing TPD related articles including sitting through a mind numbingly tedious meeting of the House of Lords.
I don’t have an answer to all of the TPD’s regs – this is merely a signpost if you like to the ones that will hit vapers and manufacturers the hardest.
And just like a review I’ll leave my final thoughts at the end – OK enough blabbering.
The EU’s Q and A on E-Cigarettes
I’ve decided to print this in full – just to show you the thinking behind the TPD and the inclusion of e-cigarettes and components in it.
You like me will see the rules leave gaping holes which as I keep saying are ‘open to interpretation’.
Why are new rules needed for e-cigarettes?
“E-cigarettes are a relatively new product category and their market share is growing.
While they may have a role to play in smoking cessation or reduction, their long-term effects on public health are not yet known.
As nicotine is an addictive and toxic substance, safety and quality requirements for nicotine-containing e-cigarettes are necessary.
Reporting obligations are also needed so that public authorities can monitor and learn more about these products.
A number of decisions on e-cigarettes will be left to the Member States, e.g. the regulation of flavours, advertising without cross border effects, and age limits.”
What will change for e-cigarette consumers and manufacturers?
Consumers of eCigarettes:
– Will benefit from improved safety and quality requirements for products: taking into account nicotine’s classification as a toxic substance, there will be a maximum nicotine concentration level for e-cigarettes and maximum volumes for cartridges, tanks and containers of nicotine liquids.
These will have to be child and tamper-proof and protected against leakage to limit the risk of exposing consumers – in particular children – to the risks of handling or ingestion.
Only ingredients of high purity may be used in the nicotine-containing liquid, and e-cigarettes will be required to deliver the nicotine doses at consistent levels under normal conditions of use.
This means that a similar level of nicotine should be delivered each time an e-cigarette is puffed for the same amount of time and with the same strength.
Will be better informed through new packaging and labelling requirements: health warnings on e-cigarette packs will be mandatory, as will instructions for their use, information on addictiveness and toxicity, a list of all substances contained in the product and information on the product’s nicotine content.
No promotional elements will be allowed on packs.
– Will be better protected: Member State authorities and the Commission will be able to act in cases of justified safety concerns relating to these products.
Authorities will monitor the market for any evidence that e-cigarettes lead to nicotine addiction or to traditional tobacco consumption, especially in young people and non-smokers, and the Commission will report on safety concerns and market developments.
E-cigarette manufacturers (in addition to manufacturing their products in line with the above rules on safety, quality and packaging) will be required to:
– Notify Member States before placing new products on the market: notification will include information on the manufacturer, the ingredients used and emissions, nicotine dose and uptake, product and production process and a declaration that the manufacturer takes full responsibility for the quality and safety of the product under normal use.
– Report annually to Member States: on the sales volumes of the products, types of users and their preferences and trends.
– Comply with specific rules on advertising: existing rules for cross-border advertising and promotion of tobacco products will also apply to e-cigarettes.
OK let’s take a look at a few things.
Maximum 10ml E-Liquid Bottles
You might have noticed this already.
More than few e-liquid companies have begun only selling 10ml or collections of 10ml bottles of e-liquid.
Those that haven’t have until May 19th to clear their stocks.
The thinking behind this is like pretty much all the regulations surrounding vaping and that is one word ‘cloudy’.
I mean by that the ‘interpretation’ of the rules are open to ‘interpretation’ if you like lol.
For instance the thinking behind this 10ml rule is I guess down to the fact that e-liquids contain nicotine.
Yeah I know not all do.
And yeah you can buy as many bottles of 10ml nicotine containing e-liquid as you like – and still buy 0mg juices in any size. Quite bizarre!
Add to that you can still legally buy VG and PG – Nicotine and concentrates to mix your own and the whole things becomes ridiculous.
And of course many e-liquid companies have now introduced nicotine ‘shots’ where you can add your chosen levels into the zero percent e-liquids!
It’s all reminiscent of Prohibition and we all know how that ended.
Nicotine and Spillage
Look, if the EU was so concerned about nicotine being such a ‘danger’ to its population then allowing it to be sold to anyone in bulk and forcing e-liquid companies to go down the ‘add it yourself’ route – has simply made the liquid more available!
Pre TPD nicotine was watered down within e-liquids.

Do you see what I’m getting at?
If the EU is concerned at spillage and the chance of nicotine getting onto the skin – then reducing the size of e-liquids means more filling and more chances of them spilling!
Add to that nicotine now being widely available in its purer form [nicotine shots etc] and you have an even greater chance of spillage of the very thing the EU is ‘worried’ about!!!
Crazy ain’t the word.
If they’re so concerned about nicotine how come they’re OK with it being used neat???!!!
Anyway the simple fact is from May 20th this year you can no longer buy any nicotine containing e-liquid larger than 10ml bottles – but you can buy a zillion of those if you want.
Talk about a colossal environmental impact with all that plastic and cardboard about.
Add to that all e-liquids [even if the recipe is changed slightly] has to go through rigorous and costly ‘testing’ [up to 6 months] to check compliance.
This could of course prove prohibitive to smaller companies forcing them out of business.
Ridiculous.
Max Nicotine Strength 20mg
To be fair that’s quite a high level of nicotine and not many newcomers to vaping and e-cigarettes let alone regular vapers will need that amount.
However it’s still unclear how this will affect sales of nicotine strengths to the DIY’ers among us.
Max Tank or E-Liquid Cartridge Set at 2ml
You can forget buying that new 5ml tank that might hit the market post May 20th – unless you shop outside the EU – as far as we can tell.
What I mean by that is and despite the EU dropping the phrase ‘cross border sales’ into the legislation – no one is completely sure how our vaping purchases from outside the EU can [or will] be policed.
The only winners I see here are China who already holds sway over the vaping industry.
Sites like Gear Best and Heavens Gifts for instance will thrive under these regulations – so long as your ‘illegal’ vape products arrive in plain brown packaging 😉
What EU Vape Businesses Need To Do
You can take a look at this page on the .Gov website for vape stores and brands in the UK that have registered to sell e cigarettes in the UK and to consumers in another EEA state (European Economic Area – the 28 EU Member States plus Iceland, Liechtenstein and Norway).
If you are a retailer not on the list we suggest registering ASAP.
Retailers, manufacturers and wholesaler also need to register with member states to be able to legally sell cross border.
Check out this table below for the EEA Member States that have either confirmed they are permitting cross-border distance sales of e-cigarettes and/or tobacco products or are yet to confirm domestic rules in this area.
| Member State | Cross-border Distance Sales of e-cigs | Website for registration/contact details |
| Czech Republic | Yes | TBC* |
| Denmark | Yes | |
| France | Yes | TBC |
| Germany | Yes | http://www.bvl.bund.de/tobacco_crossborder_registration |
| Ireland | Yes | info.tpd@hse.ie |
| Malta | Yes | TBC |
| Netherlands | Yes | https://www.nvwa.nl/ |
| Norway | Yes | TBC |
| Sweden | Yes | TBC |
| United kingdom | Yes | https://www.gov.uk/government/publications/tobacco-products-and-e-cigarette-cross-border-sales-registration |
| Slovakia | Yes | Postal address: Slovak Trade Inspection, Prievozská 32 827 99, Bratislava 27 |
| Croatia | TBC | TBC |
| Slovenia | TBC | TBC |
| Iceland | TBC | TBC |
| Turkey | TBC | TBC |
All other Member States have banned cross-border distance sales, and it would contravene the law to trade in those countries.
Keep upto date with additions and changes to the list here
*TBC (To Be Confirmed)
Businesses who intend to trade in countries where the sales confirmation, registration website or contact details are yet to be confirmed (TBC) are advised to contact the national authorities before commencing supply.
Size Matters and So Does Leaking
It’s not only the size of the tank either.
All tanks – and yes that includes RDA’s and RTA’s must be leak proof when filling and ‘tamper proof’ when in use.
This will come as a shock but this means you won’t be able to buy the latest dripper or tank from within the EU come May 20th.
Think I’m joking?
Check out the actual ‘law’:
“a product has a mechanism for ensuring re-filling without leakage if the mechanism”
- (a)entails—
- (i)the use of a refill container possessing a securely attached nozzle at least 9 millimetres long which is narrower than, and slots comfortably into, the opening of the tank of the electronic cigarette, and
- (ii)in the case of refill containers, a flow control mechanism that emits no more than 20 drops of refill liquid per minute when placed vertically and subjected only to atmospheric pressure at a temperature between 15 and 25 degrees Celsius; or
- (b)operates by means of a docking system which only releases refill liquids into the tank of an electronic cigarette when the electronic cigarette and refill container are connected.
Crazy crazy crazy!
Like I said Chinese vape superstores will think it’s Christmas as European based vapers hit their sites whilst local bricks and mortar vape shops will lose pretty much most of their best selling stocks.
Think about the effect that will have on prices – jobs and the small businesses.
Consistent Nicotine Delivery
Now this is where if you like ‘the shit gets serious’.
OK according to the EU from May 20th 2017 any new device has to go through a timely and expensive testing phase – just like e-liquids.
No bad thing you might think.
However.
All new vape devices that can legally be sold within the EU must as the TPD states:
An electronic cigarette must be able to deliver a dose of nicotine at consistent levels under normal conditions of use
Now this is a strange one that is of course open to that legal interpretation again – add lawyers to those who will do well out of the TPD.
Now taking that phrase at face value it could be ‘interpreted’ as all new vape devices – mods – tanks even mech mods – must pass a test that shows every single puff you take delivers the exact same amount of nicotine – else they’re illegal.
Now that’s just bonkers and if you read that the way I did – then that means pretty much every mod or tank – including vape pen style e cigarettes – will be illegal to sell come May 20th.
I mean sure I have 3mg of nicotine in my e-liquid and sure I have a 2ml tank on my regulated mod – BUT.
Is my little dinky Innokin Pebble with the new Aspire Nautilus 2ml tank delivering a ‘consistent level’ of nicotine every time I inhale?
Hey, sometimes I inhale for 2 seconds and then sometimes for 3.
Sometimes I shake the bottle of e-liquid before re-filling and sometimes I don’t.
You can see what I’m getting at here.
The law pretty much states if new devices and tanks can’t prove they deliver exact amounts of nicotine EVERY TIME – then they are deemed illegal.
If I’m wrong tell me – it’s just my ‘interpretation’ and I am not a lawyer 😉
Advertising – Promotion and Reviews of E-Cigarettes
When I say ‘e-cigarettes’ I am pretty much including everything to do with vaping – tanks – e-liquids and devices.
Once again we enter a kind of grey cloudy area here with the EU labelling vape stuff in the same category as tobacco – which we all know has tight rules around promotion and advertising.
When it comes to e-cigarettes the TPD rules are as I said hazy BUT the rules are left to individual countries within the EU to ‘interpret’ them as they see fit.
I’ve said more than once that here in the UK for instance the Government has taken a somewhat lighter view on how to implement them – but they will still be implemented lol.
However, and apart from a ban on TV and newspaper ads for vaping products, online promotions are now not allowed.
I guess that means we can kiss goodbye to giveaways or can we?
The fact is the rules are grey to say the least, leaving companies unclear at just what they can and can’t do.
Which leaves us with on-line reviews.
According to the UK and specifically Article 20 of the TPD when it comes to sites such as EcigClick the law is very clear:
Blogs/tweets/independently compiled, non-paid for reviews – PERMITTED
So that seems to have lifted the fog somewhat however there seems to be a school of thought among the team here that whilst we shall of course continue to conduct fair – honest and of course unpaid reviews – we might have to tone down our language a little [I think Jonny meant me lol].
In other words factual and honest is OK but for the products we really like, gushing isn’t lol
So no we aren’t going anywhere however we may need to measure our reviews slightly to the more ‘factual’ and less flowery 😉
As for e-liquid reviews we can still do those however we shall be limited to buying 0mg nicotine juices and again be careful not to gush too much or promote them as ‘healthy’.
Add to that the fact we as reviewers/bloggers/writers can’t promote e-cigarettes and vaping in general as a smoking cessation aid and you can see that even on the internet – the apparent paragon of free speech – we shall be writing with one hand tied behind out backs!
As for products supplied free of charge for the purposes of review, that would look to come under the ‘paid review’ category.
We buy 90% of the products we review here at ecigclick, it looks like we will be spending that little bit more!
Read More About the Impact of the TPD
Anyone wishing to find out more about the rules and regulations surrounding advertising and promotion of e-cigarettes and vaping should head to the UK’s Advertising Standards Agency.
They have produced a simple to read guide to the impact the TPD will have on this area.
Read it HERE
You can read the UK Government’s regulations on e-cigarettes HERE
You can also read the EU’s ‘mythbuster’ guide to exactly why and how they are legislating e-cigarettes in an infographic HERE
New Study Says E-Cig Legislation Will Turn People Back to Smoking
As the shadow of the TPD looms closer a new study says too much e-cigarette regulation will lead to more people returning to smoking.
Conducted by the Society for Research on Nicotine and Tobacco the damming findings have recently been published in the prestigious Oxford Academic University Press and should make health officials and politicians hang their heads in shame.
This was a global study looking at four countries 2 of which have relatively ‘loose’ restrictions on e-cigarette use and 2 that have stricter controls.
The UK and the United States were classed as the less controlling whilst Australia and Canada were classed as more controlling.
This is the first study looking at the impact of stronger legislation around vaping as the introduction to the findings shows:
To date, no studies have explored how different regulatory environments may influence the effectiveness of electronic cigarettes (ECs) as a smoking cessation aid.
The study was carried out via research from the International Tobacco Control survey between 2010 and 2014 and showed in follow up checks on participants that many reported after using e-cigarettes in a bid to quit smoking they had remained smoke free for at least 30 days.
Relaxed vaping Laws Mean More People Quit Smoking
The study concluded:
Use of ECs [e-cigarettes] in the real world during a quit attempt appears only effective for sustaining smoking abstinence in a less restrictive EC environment suggesting that the benefits of ECs for smoking cessation are likely highly dependent on the regulatory environment.
Bingo! There lies the main crux of the argument that e-cigarettes and vaping ARE the best smoking cessation aids out there at the present time.
However it is only in countries where vaping laws are more ‘relaxed’ that the full benefit of e-cigarettes will be felt.
But just like pretty much all the pro and anti vaping studies I’ve covered – the researchers add that MORE research needs to be carried out on e-cigarettes in general – particularly around the legislation of the industry and users:
The findings underscore the need for careful consideration on how best to regulate this emerging product so that EC benefits for smoking cessation are maximized and its risks to public health are minimized.
ECs may represent a unique and new paradigm for affecting the cessation of conventional cigarettes, although currently the effectiveness of ECs for helping smokers to quit is uncertain.
Recent meta-analytic reviews of RCTs have shown that the odds of quitting conventional cigarettes were twice as high in those using ECs with nicotine compared with those using ECs without nicotine.
Let that sink in for a moment.
People using e-cigarettes with nicotine infused e-liquid were TWICE as likely to quit smoking.
Now that represents half a billion people whose lives could be saved by the end of the century IF Governments and health officials recognized the benefits of vaping and stopped with all the draconian legislation.
At least half a billion people lives saved.
Half a billion.
Other Nicotine Replacement Therapies Slammed
Interestingly the study’s findings also includes a small piece about ‘other nicotine replacement therapies [NRTs].
This study doesn’t look at the effect of patches – gums – sprays or dangerous medication but refers to yet another study – this time from the Society for the Study of Addiction – that concluded:
Among smokers who have attempted to stop without professional support, those who use e-cigarettes are more likely to report continued abstinence than those who used a licensed NRT product bought over-the-counter or no aid to cessation. This difference persists after adjusting for a range of smoker characteristics such as nicotine dependence.
In other words all ‘approved’ NRTs are less likely to help a person quit smoking than vaping and e-cigarettes!
I’ve said it before and let’s say it again – apart from some stop smoking medication all NRTs contain nicotine – so what’s so bad about nicotine in e-liquid?
There doesn’t seem to be a week go by where I write about a new study or new piece of research around e-cigarettes.
There doesn’t seem to be a week go by where I don’t see a quote from a politician a scientist or health spokesman calling for ‘more studies’ on e-cigarettes.
I read recently there’s never been so many studies on the impact of vaping on health and smoking cessation with Dave Cross over at POTV reporting that there’s been at least 20 published since the first week of March alone this year.
Nicotine Research Bandwagon
Sometimes you have to ask yourself could all this be a smoke screen or maybe even a gravy train.
Research costs a ton of cash and generally it is private companies and corporations that fund them.
The authors of this report are quite open about receiving grants in the past from Big Pharma giant Pfizer Inc – however the funding for this research is not made clear.
They say:
KMC has received grant funding from Pfizer, Inc. to study the impact of a hospital based tobacco cessation intervention and also has served as an expert witness in litigation filed against the tobacco industry.
All other authors have no conflicts of interest to declare.
The cosying up of Big Pharma and scientific research into the health and scientific facts around vaping continues.
Whilst we as vapers should welcome positive research into vaping as a whole – we should also be aware of who funds the research.
Particularly as Big Tobacco and Big Pharma do indeed have possible ‘conflicts of interest’.
The TPD – My Final Thoughts
For the record and just like a review this is my personal view on the TPD.
I don’t care what some folks have been saying ‘that all will be well in the vaping world post TPD’ it won’t.
Up until the EU decided to put vaping under its microscope the industry was pretty much self-regulating itself and doing it extremely well.
The typical meddling from politicians and clueless scientific ‘experts’ has led to a ‘vaping spring’ none of us that vapes wants.
How dare these faceless bureaucrats and un-elected EU mandarins decide that I can’t buy a 5ml or come to that any size tank I dam well like.
How dare these faceless political none-entities decide I can only buy e-liquid in 10ml bottles.
How dare these un-elected buffoons conclude the millions up on millions of smokers who have given up tobacco for the 95% safer alternative of vaping must be wrong.
I am sick to death of this intrusive nanny state of ours and indeed it’s worldwide counterparts.
Nicotine is NOT tobacco – I’m not sure how often we have to shout this at those idiots until it gets through their thick skulls.
Look they’re even trying to say that lab produced synthetic nicotine that’s never seen a plant is ‘tobacco related’ – for God’s sake they are cretins.
Politicians and Meddling
Vaping is not only a proven and fantastic way of giving up smoking [I can still say that until May 19th] it’s also now become a pastime or hobby call it what you will – enjoyed by millions upon millions of people across the globe.
It should be lauded – embraced with the inventors and driving forces behind the movement showered with awards.
Instead these innovative lifesaving companies are being forced to jump through miles of red tape and stumping up costs that will break even the biggest of them.
It’s as if ‘they’ – the faceless politicians – have another agenda – I’ll leave you to think about what that could be.
I really hope I’m wrong about the impact the TPD will have on vaping as a whole – I really do.
But history tells us that once the politicians start regulating anything – things are never quite the same again and most certainly laws and regulations are rarely if ever repealed.
Now might be a good time to stock up on tanks and coils and in particular keep your eyes open for e-liquids on sale for silly prices as companies and sites get rid of their soon to be illegal stocks.
Bloody ridiculous.
Vape On!
Sources
- Does the Regulatory Environment for E-Cigarettes Influence the Effectiveness of E-Cigarettes for Smoking Cessation?: Longitudinal Findings From the ITC Four Country Survey
- Society for Research on Nicotine & Tobacco (SRNT)
- The International Tobacco Control Policy Evaluation Project
- Society for the Study of Addiction


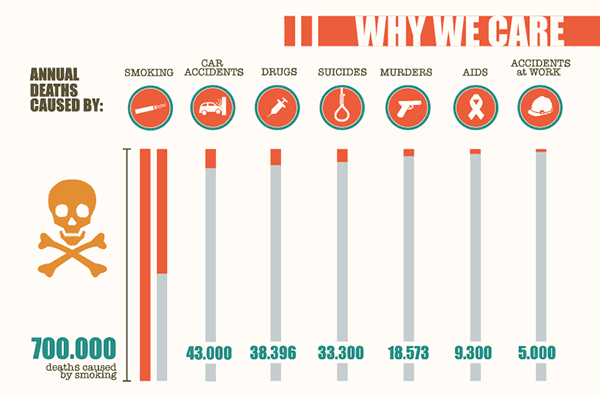









Wonder how much money the giant tobacco and cigarette companies have paid off these EU law makers into meddling and producing this kind of legislative crap. Quite clearly created to hinder the explosive growth of the Ecig industry as they are feeling the squeeze on their own profits.
I look forward to any last minute deals and one day will tell my kids the ‘golden’ age of vaping when it was free from bureaucratic nonsense.
Any sane legislator would hold discussions with the industry involved to hammer out a way forward which both served and protected the industry and the consumer. This has clearly not been the case. You do not have to be a rocket scientist to understand who and what is behind this fiasco and you can be doubly sure it has absolutely nothing to do with protecting the consumer. As with all things political it is about the money. Greed and hypocrisy abounds and we, the general public suffer as a consequence.
With hindsight, the biggest mistake the vaping industry made was adopting the descriptor ‘E-Cigarette’ the association with tobacco was never going to be a good thing and the only hope for the future will be through genuinely independent research conducted by a body beyond reproach and not funded by industries with a vested interest in obtaining the ‘right’ results.
As for the vaping industry it needs to adapt or die, there is no other choice and I am sure it will. It may be a different landscape in the future but the human race has a habit of bouncing back in the face of adversity and vaping will do the same.
So, whilst the immediate future is undoubtedly bleak I don’t think it will be terminal.
Exactly and brilliantly put – thank you 🙂